Experiment Name:
Conversion of water-insoluble Salicylic acid into water Sodium Salicylate.
Principle:
Water is a polar molecule.
It dissolved only polar solid. In pharmaceuticals, a Polarity increment may be required during several operations like liquid dosage form preparation. The increment is done by the addition of polar salt or base with various non-polar substances.
Increase the solubility of the solution with water.
Salicylic acid topical is used in the treatment of acne, dandruff, seborrhea, or psoriasis, and to
remove warts. Naturally, cucumber, broccoli, cauliflower, corn, radish, sweet
potato, and fennel contain Salicylates.
Reaction:
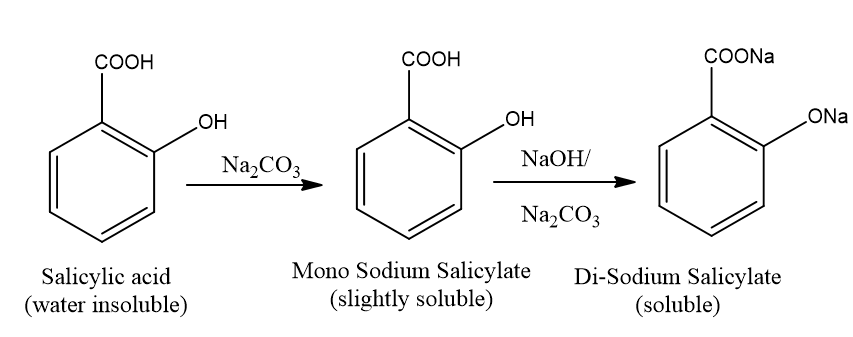
Fig.
2.1: Reaction between Salicylic acid and Sodium Carbonate.
Reagent:
§ Salicylic
acid
§ Sodium
carbonate
§ Distilled
water
Apparatus:
§ 01.Test tube
§ 02.Dropper
Procedure:
01. Take a small amount of
salicylic acid powder (< 0.5 gm).
02. Add a small amount of H2O
(approximately 1 ml) and shake
03. Observe that the powder
is not Dissolving.
04.
Now added a small amount of NaCO3 and shake well.
05.
Observed that it dissolves the Salicylic acid.
Observation:
Salicylic
acid completely dissolved in the water due to the formation of Di-sodium
Salicylate acid.
Result:
Salicylic acid-insoluble
in the water but di-sodium salicylate soluble in the water
Comment:
Salicylic
acid turned into di-sodium salicylate which was soluble in water
Precaution:
§ Have
to wear laboratory apron, hand gloves, masks, shoes & goggles.
§ Have
to be very careful during the experiment.
§ Wash
apparatus with clean water.