Section outline
-
Experiment Name: Conversion of water insoluble benzoic acid to water soluble sodium benzoate.Principle:
Water is polar molecule. It dissolved only polar solid. In pharmaceuticals, Polarity increment may be required during several operations like liquid dosage form preparation. Increment is done by addition of polar salt or base with various non -polar substance. Increase the solubility of the solution with water.
Benzoic acid helps prevent infection caused by bacteria. Benzoic acid is a topical medicine used to treat skin irritation and inflammation caused by burns, insect bites, fungal infections, or eczema .The salt and esters of benzoic acid are known as benzoates.
Reaction:
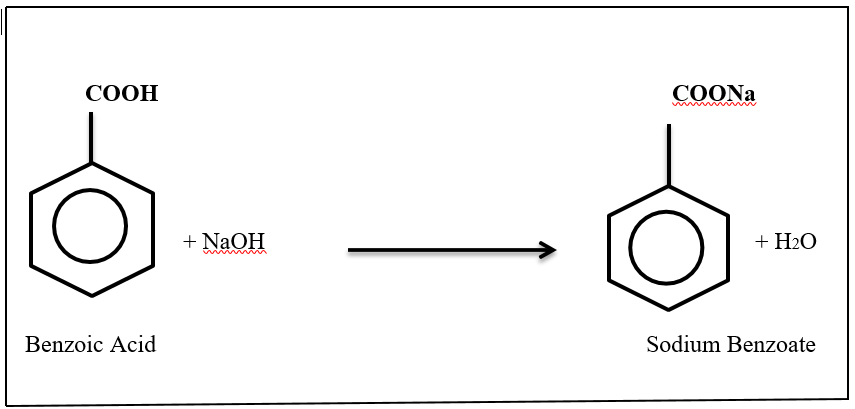
Figure 1: Benzoic acid with reacted sodium hydroxide and given product of sodium benzoate.
Required reagent for the experiment:
Ø Benzoic acid
Ø Sodium hydroxide
Ø Distilled water
Required apparatus for the experiment:
Ø Test tube
Ø Dropper
Ø Watch glass
Procedure:

Figure 2: All substance mixed in the test tube
01. Take small amount of Benzoic acid powder (< 0.5 gm).
02. Add small amount of H2O (approximately 1 ml ) and shake
03. Observe that the powder is not Dissolving.
04. Now added small amount of NaOH And shake well.
05. Observed that it dissolve the benzoic acid.
Result:
Water soluble sodium benzoate was observed.
Comment:
Benzoic acid was insoluble in water. It was added with sodium hydroxide. It produced sodium benzoate which was soluble in water.
Precautions:
Ø Have to wear laboratory apron, hand gloves, mask & goggles.
Ø Have to wear shoes during the experiment.
Ø Have to be very careful during the experiment.
