Section outline
-
Welcome to
Pharmaceutical Biotechnology

Biotechnology is one of the leading forefronts of technologies of the future. The interdependence of human health and new products together with the advent of molecular biology manipulations along with the information management system technologies at various levels of gene, transcript, and protein has generated a new research and industrial field for pharmaceutical sciences. Thus, new technologies and existing pharmaceutical requirements have come hand-in-hand to lead to new generations of biopharmaceuticals with improved activities and selective modes of action to combat diseases such as cancer, and other debilitating illnesses.
Biotechnology is the use of living systems and organisms to develop or make products, or any technological application that uses biological systems, living organisms or derivatives thereof, to make or modify products or processes for specific use. Before the development of biotechnology as an independent program, the multidisciplinary task of producing a recombinant therapeutic agent, its analysis and other related fields co-existed in various institutions. However, as the applications of this area became evident and entered into educational courses and systematic education, it became independently recognized as a program namely, Pharmaceutical Biotechnology, which could be differentiated from other fields of biotechnology such as medical, agricultural, industrial, etc. In this field, it is highly desired to, not only, design, produce and control drugs by the help of microorganisms or other biological entities, but also, to influence the healthcare system through various targeted and smart pharmaceuticals.
-
-
This chapter explores fundamental concepts, historical developments, applications in medicine, agriculture, and industry, ethical considerations, and future prospects of biotechnology in scientific advancements and innovation.
-
CONTENTS
- Biotechnology
- Sub-fields of Biotechnology
- Pharmaceutical Biotechnology
- Genetic Engineering
- Difference Between:
Biotechnology & Pharmaceutical Biotechnology
Biotechnology & Genetic Engineering - Application of Biotechnology
- Role of Pharmacists
-
-
This chapter explores recombinant DNA technology, highlighting gene cloning, expression systems, and biotechnological production of pharmaceuticals, enzymes, and genetically modified organisms for medical, industrial, and agricultural applications.
-
CONTENTS
- Basics of DNA
- The
Central Dogma
Replication
Transcription
Translation - Recombinant DNA Technology
- Preparation of Recombinant DNA
-
Do you want to know more about the central dogma of molecular biology?
Then please go through this handnote which I have prepared for you! :)
-
CONTENTS
- Preparation of Recombinant DNA
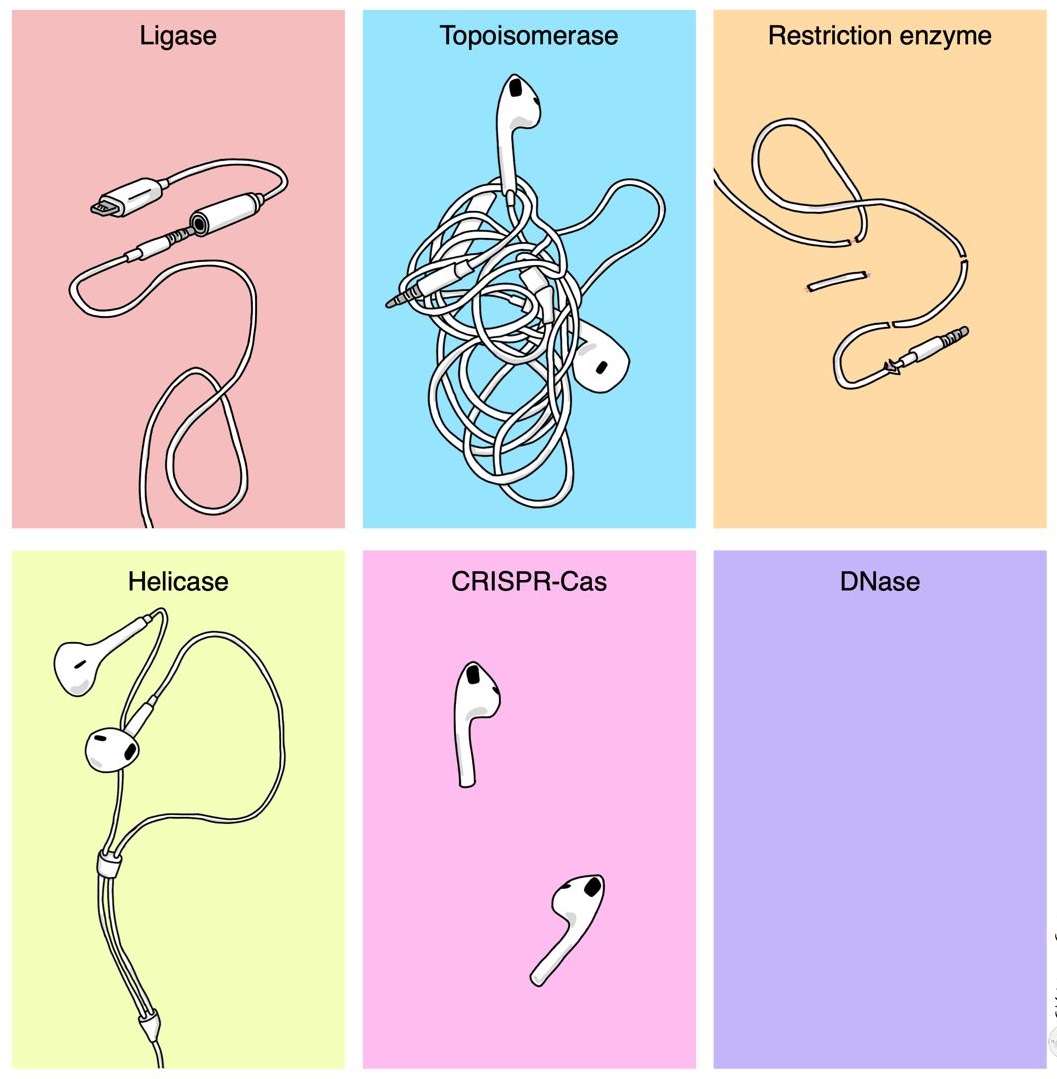
- Enzymes & Restriction Enzymes
- Naming Restriction Enzymes
- Restriction-modification System
- Recognition Site & Restriction Site
- Vectors
- Introducing Recombinant Vector
-
Why is it so important to have the ampR gene and lacZ gene in a plasmid?
This handnote will try to answer all your questions which you had in the class! ;)
-
CONTENTS
- DNA Amplification (PCR)
- DNA Fractionation (Gel Electrophoresis)
- DNA Analysis (Blotting)
- DNA Fingerprinting
-
-
This chapter covers stability, excipients, delivery systems, and regulatory considerations for biologics, ensuring efficacy, safety, and shelf-life optimization in pharmaceutical development.
-
CONTENTS
- Biotech Products
- Formulation of Biotech Products
- Excipients Used in Biotech Products
- Microbial Considerations
- Shelf-life Considerations
- Route of Administration Considerations
-
-
This chapter explores strategies for delivering protein-based drugs, addressing stability, bioavailability, controlled release systems, enzymatic degradation, absorption enhancement techniques, and advanced delivery methods like nanoparticles, liposomes, and transdermal formulations.
-
CONTENTS
- Proteinous Drug Delivery
- Advantages of Proteinous Drug Delivery
- Problems Associated with Proteinous Drug Delivery
- Overcoming the Challenges of Proteinous Drug Delivery
- Site-specific Carrier Molecule in Targeted Drug Delivery
- Ringsdorf’s Model
-
-
This chapter involves the introduction, removal, or alteration of genetic material within a patient's cells to treat or prevent diseases, utilizing viral and non-viral vectors for targeted genetic modifications.
-
CONTENTS
- Gene Therapy
- Gene Therapy Based on Cell Types
- Gene Therapy Techniques
- Gene Therapy Approaches
- Gene Delivery Systems
- Transgenic vs Knockout Animals
- Problems Associated with Gene Therapy
-
-
The chapter covers biopharmaceutical production, including fermentation, cell culture, purification, formulation, quality control, regulatory considerations, and Good Manufacturing Practices (GMP) for safe and effective biotech drug development.
-
Human Insulin Production: The hormone insulin is essential for the control of blood sugar levels. Diabetes mellitus is a disease in which some people cannot make insulin themselves. This disease kills many people in the world every year.
Hepatitis B Vaccine Production: Hepatitis B (HB) is one of the most common infectious diseases known to man. Recombivax HB was approved as a hepatitis B prevention vaccine in July 1986. Using recombinant DNA technology, Recombivax HB uses the surface antigen of the virus that stimulates the production of protective antibodies which combat the HB virus.
-
-

- The presentation will be held in the designated class (check the schedule).
- The presentation will be held in a group. Each group will have a maximum of 15 minutes to present. However, there is no minimum time limit.
- After the presentation, each group will have to face a 5 minutes Q/A session. Faculty, as well as other students, will ask the presenters questions.
- You can use a maximum of 15 slides in your presentation. However, there is no minimum number of slides.
- Slides are mandatory for the presentation. Simultaneously, you can also use the whiteboard/marker.
- Your group members and the topic will be provided here in due time. So please do not forget to have a look here from time to time (check the time and topic here).
- You may be asked to present from anywhere on the slide. So, please be prepared on the day of your presentation and have a complete idea of your whole topic.
- You will get a mark as an individual, based on eight parameters.
- A presentation is as good as its audience. So make sure to pay full attention when your peers present their slides. Chances are incredibly high that you will be asked questions from their slide if I feel that you are not attentive in the class (and it will carry marks according to the rubrics).
- Some tips to get a better grade in the presentation:
- Wear formal
- Speak clearly in English
- Try to start your presentation with a hook (asking a question to the audience and engaging them)
- Do not read your slides or make your audience read your slides either! Rather, speak as it is a story
- Make your slides informative and graphical. However, do not put too much text in your presentation. Use bold and bullet points if required
-
-

Assignment Submission Guideline
-
Convert your PPT file to a PDF. Make sure the PDF file size is under 10 MB.
-
Rename the PDF file using your ID number (e.g., 213-29-001).
-
Submit the PDF in the BLC, noting that only one submission is allowed.
-
-




